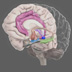
Beating Stressful Memories
New research showing how memories take shape may lead to better treatments for unwanted memories as well.
New research showing how memories take shape may lead to better treatments for unwanted memories as well. Building on research dating to the 1990s and farther back, studies today are showing specific ways to reduce the fear-laden portion of traumatic memories, such as those that occur in post-traumatic stress disorder. Some approaches center on stress hormones, which help to entrench memories of emotionally significant events. For example, epinephrine and cortisol, both released by the adrenal glands, act on receptors in the brain to sear the memory in place, quickly and often indelibly. “Stress hormones make stronger memories,†says Jim McGaugh of the University of California, Irvine. McGaugh’s early work showed that epinephrine helps rats form a quick association between a place and a mild shock. Usually rats given this conditioning will refuse to re-enter the place, expecting the shock to follow. But when given the drug propranolol, which blocks epinephrine receptors, before training, the animals show no alarm. In a classic experiment published in 1994 in Nature, McGaugh teamed up with Larry Cahill, also at Irvine, to study the effects of propranolol on emotional memories in humans. Volunteers listened to a story with a tragic ending or one with a similar, but emotionally neutral, plot. Propranolol impaired memories for the tear-jerker but not for the neutral story, indicating that the epinephrine released during the emotional scenes otherwise would have intensified the memory. McGaugh’s work led other researchers to pick up on the implications for PTSD. Cahill, Roger Pittman of Harvard University and their colleagues published a study in 2002 in which victims of traumas, including car accidents, rape, and medical emergencies, received propranolol or a placebo within six hours of arriving at the hospital and continuing for 10 days. When tested three months later, almost half of the placebo group showed physiological signs of PTSD in response to questions about the traumatic event; however, none of the propranolol-treated patients did. This study, published in Biological Psychiatry, also demonstrates that that by stanching the flow of epinephrine, the intervention prevented what might have become a barrage of unbearable memories. The work has raised ethical concerns. A 2003 report released by the President’s Council on Bioethics warns that society is ill-served if memory can be tampered with. But McGaugh emphasizes that propranolol given soon after a trauma does not wipe out memory or obscure the facts about what happened. Rather, it blocks epinephrine receptors in the amygdala, a part of the brain that supports the emotional component of memory. Factual details, such as what happened, when, and where, are processed in another area, the hippocampus. “Beta blockers mute the memory’s intensity and prevent it from dominating the person’s thoughts at a later time,†McGaugh says. The Reconsolidation Question Blunting the force of a memory while it is being formed affects the pathway through which an experience enters long-term storage. During this “consolidation,†new proteins are produced to build additional synapses, or points of connection between neurons, that support the memory—presumably making it a fixed entity. But some research suggests that even long-term memories are amenable to change. According to the “reconsolidation†hypothesis, retrieving a previously formed memory can put it back into a transient, state, where it again requires new proteins to be restabilized. Studies have hinted at reconsolidation for decades. In the August 17, 2000, issue of Nature, Karim Nader, then working in Joe LeDoux’s lab at New York University, along with LeDoux and Glenn Schafe, provided evidence that many researchers found convincing. The team trained rats to associate a tone with a mild shock; 24 hours later, the rats heard the tone and immediately received an infusion of anisomycin, which blocks protein production, into the amygdala. These animals quickly lost their fear of the tone—as evinced by their lack of a freezing response. This memory deficit was not seen in animals that did not get a “reminder†tone later, showing that the undisturbed memory was stored and thus impervious to anisomycin. Nader, now at McGill University, says the study shows that the memory being played out (hearing the tone soon after the initial learning, in the rats’ case) opens a window of opportunity during which the memory can be modified. Because protein-synthesis inhibitors such as anisomycin do impair memories, this approach would not be a treatment option. But propranolol is a possibility. Nader and Alain Brunet, also at McGill, and Pittman at Harvard have concluded a study of 20 patients with PTSD, of whom half were given propranalol during supervised recall of their trauma, often many years after the event. The study is in press with the Journal of Psychiatric Research and has been covered on the television show “60 Minutes.†Patients given propranolol, when asked about their trauma, showed physiological responses in the normal range, not the spikes in heart rate and respiration that are typical of PTSD. Most of the treated patients felt better, but not significantly better than the placebo group. Nader speculates that someone who has had PTSD for 20 years will probably need more than one treatment session to notice a difference. Still, he finds the results encouraging: “We may be on the threshold of intelligently affecting memory systems that have been in place for many years.†Nader adds that the work has broader implications. “In PTSD, for example, memories are stored with an intensity that’s set way too high—the mechanisms that normally regulate them are overwritten,†he says. Blocking reconsolidation may be a way to reset the system in other forms of inappropriate learning, such as drug addiction. Barry Everitt and colleagues used a compound that blocks a key gene in memory formation in the amygdala. Working with rats that had formed longstanding cocaine addiction, the investigators showed in the September 15, 2005, issue of Neuron that an infusion of the compound at the same time as a light associated with cocaine was enough to eliminate drug-seeking behavior. LeDoux and Nader’s initial report in 2000 triggered a flood of studies examining reconsolidation in many kinds of memory, and in many species. Their research also has its share of skeptics, some of whom describe reconsolidation as a controversial hypothesis. Says Yadin Dudai of the Weizmann Institute of Science in Rehovot, Israel, “The data show that a memory trace can enter into a unique altered state during retrieval, resembling, though not faithfully recapitulating, the consolidation period after the memory is encoded. This ‘reconsolidation’ occurs under specific conditions that are only partly understood.†The upshot, says Dudai, is something cognitive scientists have known for many years: “Long-term memory is more unstable than we tend to assume.†Making Fear ‘Extinct’ Another way to make a memory more manageable is to enhance the process by which we reduce our fear of bygone events—“extinction,†in scientific parlance. For example, rats conditioned to associate a tone with a mild shock will eventually lose their fear if they hear the tone often enough without the shock. The rats do not forget the shock; rather, they form a new memory in which the tone now has no consequences. “Extinction is not memory erasing,†says Michael Davis of Emory University. “It’s a new form of learning that acts to inhibit the original fearful memory.†Some forms of psychotherapy involve recalling the traumatic event with the support of a therapist, sometimes along with anti-anxiety medications or antidepressants. But Davis notes that these drugs only treat the symptoms of anxiety. In a study published in the November 2004 Archives of General Psychiatry, Davis and colleagues examined whether a drug that improves extinction in animals would also improve the effectiveness of exposure-based psychotherapy. The investigators homed in on the NMDA receptor, a specific type of receptor for glutamate, one of the brain’s chief chemical messengers. Previous research had shown this receptor to be pivotal in the extinction process. The 2004 study used a drug called D-cycloserine, or DCS, which intensifies the actions of the NMDA receptor. Seventeen individuals in a group of 27 patients with acrophobia (the fear of heights) took DCS at one of two doses, in tandem with two trials of a standard exposure-based therapy—a virtual-reality helmet that made them feel they were peering over a high railing. Ten others received the virtual reality therapy with a placebo. Three months after the sessions, participants who received DCS felt significantly better than the placebo group. When looking over the virtual edge, the treated group reported feeling less anxious and showed significantly decreased skin fluctuations (an objective measure of anxiety) compared with those who took the placebo. They also tackled real-life heights more readily than controls. Other researchers have identified the brain’s cannabinoid receptor as a key player in fear extinction. Beat Lutz and colleagues at the Max Planck Institute in Munich, Germany, have showed that “knockout†mice lacking a functional cannabinoid receptor are unable to extinguish fear-related memories. Lutz and colleagues showed in the April 14, 2007, issue of Endocrinology that in mice lacking this receptor, the entire stress response is skewed. Addressing fear at the level of extinction is a different approach from the reconsolidation work, Davis says—one that does not lessen the impact of the original memory but, rather, boosts the brain’s natural ability to form a new memory of the same event, minus the emotional freight. Interventions such as these will not remove from memory key portions of people’s lives, but they may help prevent the past from crippling the present. While it is true that those who cannot learn from history are condemned to repeat it, treatments may be on the horizon for those who have learned their traumatic past all too well.
memo, memory, ptsd, hippocampus, amygdala, stress, traumatic, traumatic stress disorder, post traumatic stress disorder, epinephrine, cortisol
- ID: 850
- Source: DNALC.G2C
Related Content
2211. Resilience, stress, and plasticity
Professor Bruce McEwen describes the interplay between reilience and stress, which can cause the brain to shrink or grow.
2289. The Amygdala and Posttraumatic stress disorder (PTSD)
Doctor Daniel Pine explains that the amygdala is involved in learning to respond to a fearful experience fear-learning. There is evidence that the same response can lead to PTSD.
810. Stress and Brain Development
Professor Pat Levitt discusses how stress affects the biochemistry of the brain and plays a major role in most cognitive disorders.
821. Stress and Brain Development
Professor Pat Levitt discusses how stress affects the biochemistry of the brain and plays a major role in most cognitive disorders.
849. Hippocampus and Memory (2)
The potential gains of improving or therapeutically altering memory are compelling, but ethical considerations are imperative.
2082. Depressed learning
Professor Wayne Drevets discusses specific types of learning deficits associated with depression. These may be caused by biochemical impairments in long-term potentiation.
822. The Amygdala, the Body's Alarm Circuit
The amygdala controls autonomic responses associated with fear, arousal, and emotional stimulation and has been linked to anxiety disorder and social phobias.
825. A Brain Built for Fair Play
A new theory of the neuroscientific basis for the human instinct for fair play.
2223. Bipolar disorder
An overview of bipolar disorder-related content on Genes to Cognition Online.
2103. Limbic System
The limbic system is a group of brain structures including the amygdala, hippocampus, and hypothalamus that are involved in processing and regulating emotions, memory, and sexual arousal.